 The UWPKDAP (University of Washington Program on Pharmacokinetics of Drugs of Abuse during Pregnancy) is an NIH (NIDA)-funded research program in the Department of Pharmaceutics, School of Pharmacy at the University of Washington.
The UWPKDAP (University of Washington Program on Pharmacokinetics of Drugs of Abuse during Pregnancy) is an NIH (NIDA)-funded research program in the Department of Pharmaceutics, School of Pharmacy at the University of Washington.
The primary purpose of the program is to elucidate the fundamental mechanisms of maternal-fetal drug disposition in pregnant women with special emphasis on drugs of abuse and treatment of drug abuse.
These data will be synthesized into Physiologically Based Pharmacokinetic (PBPK) models that will help predict maternal and fetal exposure to illicit and licit drugs used by pregnant women. The program, led by Dr. Jashvant Unadkat, consists of three projects.
Project 1
 (Co-PIs: Drs. Jashvant Unadkat and Qingcheng Mao (dec.); Co-investigator: Dr. Ed Kelly):
(Co-PIs: Drs. Jashvant Unadkat and Qingcheng Mao (dec.); Co-investigator: Dr. Ed Kelly):
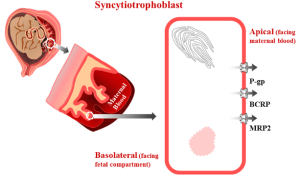
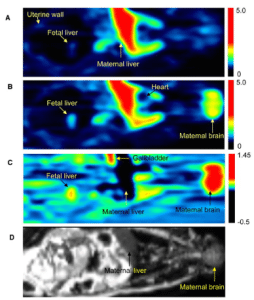
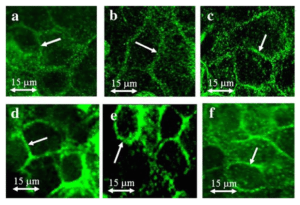
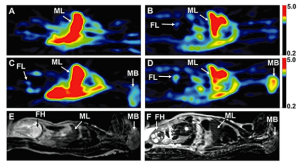
This project aims to 1) identify drug-metabolizing enzymes (DMEs) and quantify the fraction metabolized (fm) and intrinsic metabolic clearances of THC/11-OH-THC in maternal, placental and fetal tissues using tissue microsomal and cytosolic fractions; 2) identify transporters and quantify the fraction transported (ft) and intrinsic transport clearances of THC/11-OH- THC in human placentae and maternal tissues (e.g. liver) using transporter- transfected cells (Aim 2a1) as well as the perfused human placenta; and 3) confirm that the in vivo fetal exposure (including fetal brain exposure) to THC/11-OH- THC, and its magnitude, is modulated by P-gp and/or BCRP in wild-type, P-gp-/-, Bcrp-/- and P-gp-/-/Bcrp-/- mice.
Current Students/Postdocs: Xin Chen and Ankit Balhara
Project 2
(PI: Dr. Nina Isoherranen):
This project aims to 1) demonstrate that estradiol and cortisol induce THC, 11-OH-THC and 11-nor-COOH-THC clearance in human hepatocytes and intestinal mucosa, and that fatty acid binding proteins (FABPs) play a critical role in directing metabolism of THC, 11-OH-THC and 11-nor-COOH-THC, including characterization of the intestinal-liver interplay in THC metabolism and to quantify the respective effects of estradiol and cortisol on THC metabolism and use the collected data from aim 1 and aim 2 to predict and model the effects of pregnancy on THC metabolome and assessment of THC exposures its regulation by pregnancy hormones; and 2) establish that THC clearance is induced in vivo by estradiol and cortisol and that this induction can be predicted using in vitro data through a clinical study.
Current Students: Aurora Authement, King Yabut, Keiann Simon
Project 3
(PI: Dr. Jashvant Unadkat); Co-investigators: Dr. Lyndsey Benson, Dept of OBGYN, Univ. of WA, Dr. Erica Wymore, Dept of Pediatrics, Univ. of Colorado)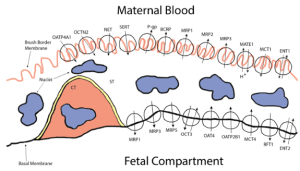
This project aims to 1) extend and refine our current m-f-PBPK model to allow prediction of maternal-fetal-placental exposure to THC/11OHTHC (including fetal brain) after oral and inhalational use of marijuana by a) extending the model to include THC administration by inhalation; and b) populating the model with system- and drug-dependent parameters; verify the m-f-PBPK model, collect and determine THC/11OHTHC concentration in the following samples from pregnant women who regularly use marijuana: (a) umbilical venous (UV) blood, maternal venous blood and placenta at delivery (third trimester); (b) fetal tissue (e.g. brain), placental tissue and maternal venous blood from elective terminations conducted during the first two trimesters; and 3) utilize our refined m-f-PBPK to predict and verify maternal-fetal-placental exposure to THC/11OHTHC (including fetal brain) after oral and inhalational use of marijuana (using data from Aim 2); b) conduct an exploratory –omics study to ascertain “molecular signatures” of THC neurodevelopmental toxicity using fetal brain tissue from women who regularly do or do not use marijuana.
Students: Aditya Kumar