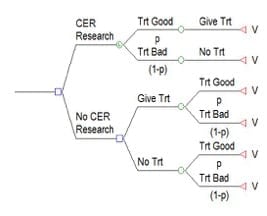
Value of Information for Cardiovascular Trials and Other Comparative Research (VICTOR) is a web-based platform that can help researchers planning a comparative cardiovascular disease study using clinical trial or other research designs to estimate the potential value of their study. Such estimates may be useful in justifying the significance of a proposed study, assisting in research prioritization, or even selecting study designs. The valuation technique follows standard Bayesian value of information (VOI) methods. VICTOR computes VOI estimates based on inputs that are gathered in a research proposal that the investigators submit to a funder. VICTOR combines this proposal-specific clinical information with disease progression models, predictive models, US population sizes, and mortality rates. A variety of cardiovascular endpoints can be selected. The value of the study is expressed in terms of life years gained at a population and the per-patient level.
These estimates of value can be used convey the importance of research to prospective funders. Funders can also use this platform to better understand the portfolio of research that they are funding.
VICTOR is development based on collaborative work between The CHOICE Institute and the Department of Cardiology at the University of Washington, Seattle. This work is supported by an research grant from the National Health, Lung, and Blood Institute (R01HL126804).
Resources on VOI
- Conceptual ideas behind VOI
- How can clinical researchers quantify the value of their proposed research? -Basu et al. American Heart Journal (2019)
- ISPOR Task Force on VOI – Part 1 – Introduction, Fenwick et al. Value In Health (2020)
- ISPOR Task Force on VOI – Part II – Analyical Methods, Rothery et al. Value In Health (2020)
- Claxton et al. 2013, CHE Research paper 83
- Selected Literature on VOI
- International Consortium on Value of Information
VICTOR Documentation
- Minimal Modeling, Life-Expectancy Calculations, and Validations in VICTOR
- A Systematic Review of Heart Failure Trial Endpoints
- User Guide and Empirical Example on SHEP Trial
- User Guide and Empirical Example on ALLHAT Trial
- User Guide and Empirical Example on ACCORD trial
- Use Guide and Empirical Example on AIM-HIGH trial
Using VICTOR Platform
- Feasibility questionnaire – Is your research eligible for VOI calculations using VICTOR?