For the first time, researchers have images showing how a virus pries its way past host membranes

Three to five million people worldwide suffer from the flu each year—and it’s deadly. The World Health Organization (WHO) estimates that 250,000 to 500,000 people a year die from the flu. The people most at risk are those with compromised immune systems: children, older adults, people with immunosuppressive illnesses, and the unvaccinated.
But if you get the flu, those numbers just don’t seem to matter. You feel terrible—like you’ve been slammed by a giant bag of concrete. You lose strength and collapse into bed with fever, chills, aches, pains and more. Your body is all out of sorts. You feel like a zombie. Total dysfunction.
When you have the flu, you are out of sorts—right down to the molecular level.
Thanks to some advanced electron microscopy from UW School of Pharmacy’s Associate Professor of Medicinal Chemistry Kelly Lee’s laboratory, we are starting to understand how the influenza virus aggressively pries its way into cells. The team’s research may eventually lead to improvements in prevention and treatments for the flu and other similar viruses like HIV, herpes, dengue and Zika—diseases that affect millions worldwide every year.
The Journal of Virology published this break-through study in which, for the first time, Lee’s team captured 3-dimensional nanoscopic images of the molecular events that the flu virus carries out in order to fuse itself with a host cell and start a new infection. To create the 3D images, the team is riding a wave of new applications of cryo-electron microscopy (cryo-EM). These powerful electron microscopes, and the next generation cameras they use, are making it possible to obtain unprecedented glimpses into structures of protein machinery and viruses.
They take the protein and virus in a buffer and without fixing or staining the specimen, flash freeze it by plunging the specimen into cryogenic liquid ethane. With that kind of cold, the sample freezes before the water can crystallize, which preserves the proteins and membranes in their native states. Like in an X-ray CAT scan, they tilt the samples to get different views of the viruses undergoing fusion and then use powerful computational methods to reconstruct the 3-dimensional image.
Flu viruses are “enveloped” viruses, and like HIV, herpes, dengue and the Zika viruses, they have a lipid membrane that protects their genetic material. To deliver its genome into cells, the virus needs to merge this membrane “envelope” with the host’s cellular membrane. With better insight into the mechanics of this process, researchers may gain an understanding of how to interfere with this stage of cell entry and prevent infection. Indeed, antibodies produced by our immune systems target and in some cases jam the protein machinery that viruses use to gain entry into cells.
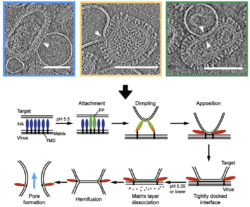
Flu viruses are covered in spikes. Their external membranes are decorated with fusion protein, known as “spike complexes”— because they look just like that – little spikes arrayed across the virus’ surface. As the virus makes its way through your body, those spikes make contact with healthy cells. They grapple onto the membrane of the healthy cell (or target) and then draw the target membrane towards the virus, creating a dimple in the target surface. The spikes then attach, or pinch, the membrane and draw it closer to the virus, not unlike a tractor beam out of science fiction. When the target membrane is pulled tight, or docked, to the surface of the virus, there is a fusion that happens between the cell’s membrane and the virus’ membrane. These cell and viral leaflets join together to open a channel through which the virus delivers its genome to the cell.
This breakthrough study allowed the team to look at the different stages of the membrane fusion event and figure out the sequence in which they occur. Prior to this, “The field had a lot of indirect information from lower resolution techniques but now for the first time we are able to look at these processes at pretty high resolution and see the proteins, the membranes and how they become contorted and remodeled as the virus carries out membrane fusion. Most of the structures we see for membranes and how they get deformed are somewhat unexpected. It’s surprising and exciting when you see how these events are taking place at the molecular level,” said Lee.
The team’s research continues. “We hope go further in our research with enveloped viruses and are working with other departments to bring more electron microscopy resources to the UW,” shared Lee. “There is strong demand among researchers in many areas of biological sciences to use these types of microscopes and harness their capabilities to help us understand cellular processes at the nanoscopic level.” Beyond enveloped viruses, membrane fusion also is at the heart of basic cell biology and is a critical step in sperm-egg fertilization and signaling across nerve synapses, for example. The Lee lab hopes that their work on the influenza virus case may also help to improve the understanding of these fundamental functions in the life of cells.
Also: UW Health Sciences NewsBeat Lab uses electron microscopy to visualize flu virus invasion: Pharmacy team’s images are first to capture virus infecting healthy cell